Legionella & ST108 Testing Services
Q Laboratories has over 50 years of experience in water testing. Currently we specialize in providing cultural and rapid detection test methods for Legionella monitoring, ST108 Compliance, and connecting the Water Treatment Industry with the most current technology with the most current technology to support water management programs.
We are proud members of the CDC Elite Program and the Association of Water Technologies (AWT), and honored to be recognized with expert lab status by AOAC, MicroVal, and AFNOR.
Legionella Test Offering
Legionella testing is the analytical process of detecting and quantifying Legionella spp. in water systems to verify the efficacy of water management programs. Q Laboratories’ testing methods include Cultural Method (ISO 11731), qPCR, and PCR. These analyses are commonly performed on potable and non-potable water sources such as cooling towers, domestic hot and cold systems, and decorative fountains, with results guiding remediation and control strategies in healthcare, industrial, and commercial settings.
qPCR
- Quantification of live Legionella spp., L. pneumophila SG-1, L. pneumophila SG 2-15.
- Results delivered the next day!
Cultural Method
- Rapid identification via Bruker Maldi-TOF technology included
- Allows for confirmation of Legionella recovery with speed and confidence, eliminating analyst variability
- Accurate, reliable results delivered within 7-10 business days
PCR
- Qualitative method confirming presence or absence of Legionella spp.
- Limit of Detection – 10 CFU/L
- Results delivered within 2-3 business days
ST108 Offering
ANSI/AAMI ST108 provides guidance to medical facilities and water treatment professionals alike on how to best protect patients from Hospital-Acquired Infections through effective water management. Q Laboratories focuses on supporting you in adhering to this new standard through a rigorous ST108 testing program to ensure all Joint Commission accreditation standards and any future third-party requirements are met.
Annual Validation
- Ionic Analysis
- HPC Bacterial Counts
- Total Hardness
- Color
- Turbidity
- Alkalinity
- Endotoxin
- pH
- Conductivity
Monthly Critical Water
- HPC Bacterial Counts
- Total Hardness
- Alkalinity
- Endotoxin
- pH
- Conductivity
Quarterly Utility Water
- HPC Bacterial Counts
- Total Hardness
- Alkalinity
- pH
- Conductivity
Quarterly Steam
- Alkalinity
- Total Hardness
- pH
- Conductivity
AAMI ST108 Sampling Instructions:
- Select the sample location within the water distribution system.
- Sanitize the tap with an alcohol-based disinfectant. Do not use bleach or other disinfectant solutions. Allow the tap to air dry completely.
- Open the sample tap fully and allow the water to flow for at least one (1) minute. Reduce the flow to about the width of a pencil to allow the bottle to be filled without splashing.
- Do not rinse out the bottle prior to collecting the water sample. Use aseptic techniques when collecting the water to avoid contaminating the cap or bottle.
- Collect the water sample:
- Write the sample location on the sample bottle.
- Grasp the bottom of the sample bottle with one hand and remove the cap. Hold the cap by the exterior only and take care not to touch the mouth of the bottle or inside of the cap with fingers or the sample could become contaminated.
- For HPC and endotoxin testing fill the bottle to within ½-1 inch from the top.
- For all other testing fill the bottle completely, leaving no headspace.
- Immediately recap the sample bottle tightly and store at refrigeration temperature until delivery to the testing laboratory.
For each sample submitted for ST108 compliance testing, the sampling date must be clearly documented by the client on the submission form. This information is critical for ensuring compliance with regulatory holding time requirements. If a sampling date is not provided at submission for each sample, Q Laboratories will proceed with analysis as submitted. If the sample date is provided and is received is outside ST108 guidance for hold time, the submitter will be notified by email. Testing cancellations must be requested within 60 minutes of email notification. In such cases, ST108 compliance cannot be guaranteed, and the client assumes full responsibility for any resulting implications.
What is the CDC ELITE program?
This acronym stands for the Centers for Disease Control Environmental Legionella Isolation Techniques Evaluation. The program allows laboratories to test their Legionella isolation techniques against a set of standardized samples. The labs that correctly identify Legionella in 2 consecutive panels receive documentation for passing the proficiency test. Since 2013, Q Laboratories has been continuously recognized as a CDC ELITE Certified laboratory.

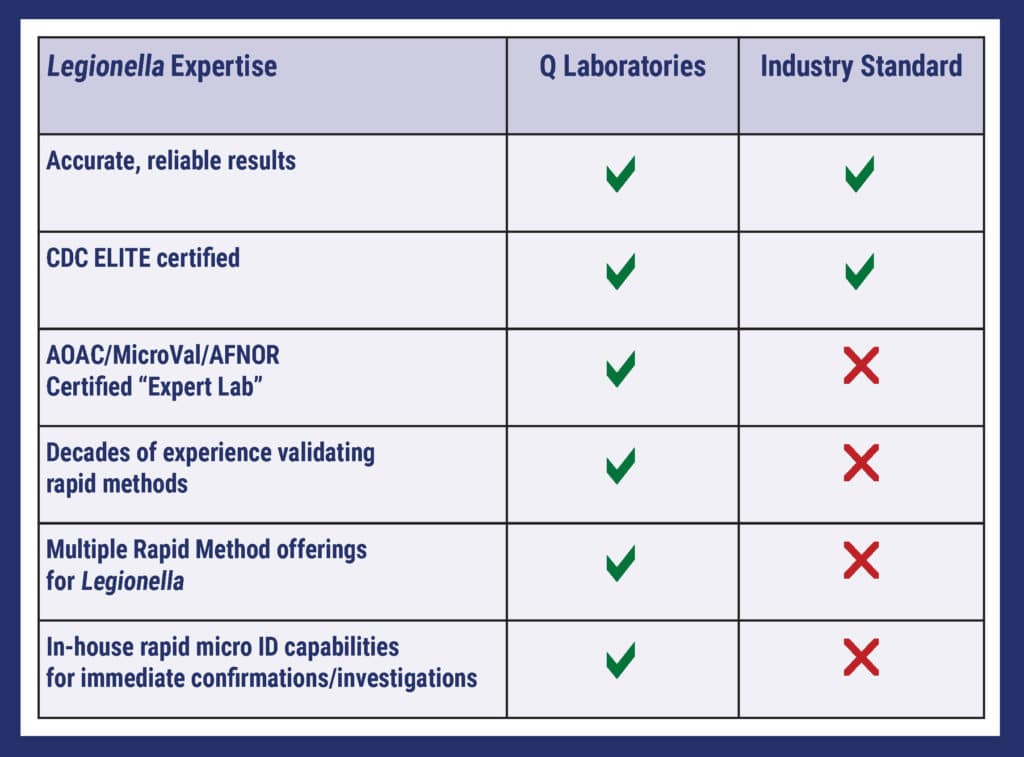
Compare Q Labs to the Industry Standard
Read Our Paper:
“Comparative Evaluation Of Three Modern PCR Methods For Quantitative And Qualitative Analysis Of Legionella spp. For Routine Monitoring Of Premise Water System Samples.”


